Are you curious about how water gets purified or how certain chemicals are separated so efficiently? The secret often lies in ion exchange resins.
But did you know there are different types of these resins, each designed for specific tasks? Understanding these types can help you choose the right solution for your needs—whether it’s for water treatment, pharmaceuticals, or industrial processes. Keep reading, and you’ll discover the key differences and how each type works to make your processes cleaner and more effective.
This knowledge could change the way you approach your next project.
Basics Of Ion Exchange Resins
Ion exchange resins are small beads made from organic polymers. These beads carry charged groups that attract ions of opposite charge. The resins swap ions in a solution with ions attached to their surface. This swapping process is called ion exchange.
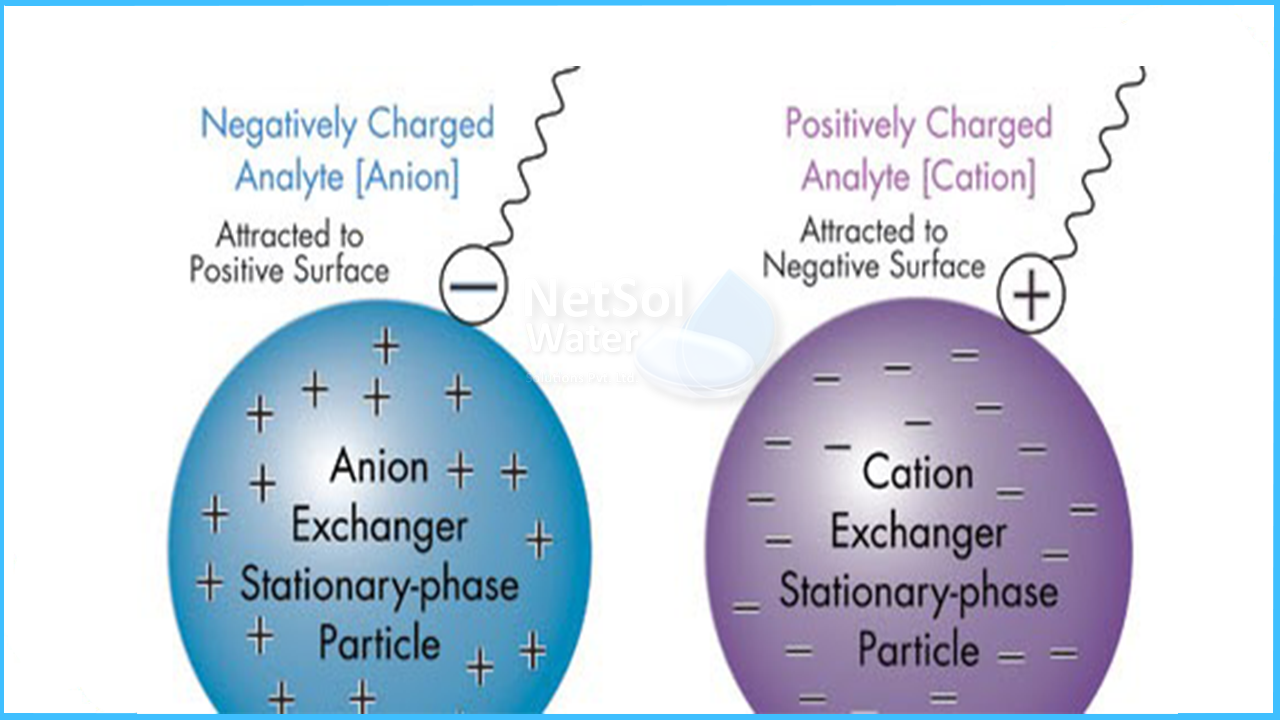
These resins come in two main types: cation exchange resins and anion exchange resins. Cation resins attract positively charged ions, while anion resins attract negatively charged ions. This ability makes them useful in many industries and processes.
What Ion Exchange Resins Do
Ion exchange resins remove unwanted ions from liquids. They replace harmful ions with safer ones. For example, they can remove calcium and magnesium from water to soften it. The resins capture these ions and release sodium or hydrogen ions instead. This process improves water quality and protects equipment.
Besides water treatment, resins help in separating chemicals. They bind specific ions and release others, making purification easier. This action is vital in chemical manufacturing and pharmaceutical production.
Common Applications
Water softening is a common use of ion exchange resins. They protect pipes and appliances from scale buildup. Resins also clean wastewater by removing heavy metals and toxins. This helps industries meet environmental rules.
Food and beverage industries use resins to purify products. They remove unwanted minerals and improve taste. In medicine, resins help in drug purification and dialysis. Their role is essential in many daily processes.
Classification By Ionic Charge
Ion exchange resins are widely used in water treatment and chemical processes. They are mainly classified by the type of ionic charge they carry. This classification helps to understand their function and application better. The two primary types are cation exchange resins and anion exchange resins.
Cation Exchange Resins
Cation exchange resins carry a negative charge. They attract and hold positively charged ions, called cations. Common cations include calcium, magnesium, and sodium. These resins are used to soften water by removing hardness ions. They replace hard ions with sodium or hydrogen ions. This process improves water quality for many uses.
Anion Exchange Resins
Anion exchange resins carry a positive charge. They attract and hold negatively charged ions, called anions. Typical anions include chloride, sulfate, and nitrate. These resins remove unwanted anions from water or solutions. They help in processes like water purification and wastewater treatment. Anion resins are vital in removing harmful substances.
Strong Vs Weak Resins
Ion exchange resins play a key role in water treatment and chemical processes. They come in two main types: strong and weak resins. Each type works differently and suits specific applications. Understanding the difference helps choose the right resin for your needs.
Strong Acid And Base Resins
Strong acid resins have very acidic groups. They fully release hydrogen ions. These resins work well in hard water softening. They remove calcium and magnesium efficiently.
Strong base resins contain strong basic groups. They fully release hydroxide ions. These resins are good for removing anions like chloride and sulfate. They work well in water purification.
Weak Acid And Base Resins
Weak acid resins have partially acidic groups. They release hydrogen ions less completely. These resins work best in softening water with fewer hardness ions. They are gentle and selective.
Weak base resins have weak basic groups. They release hydroxide ions partially. These resins remove specific anions like nitrate and carbonate. They work well in special water treatments.

Credit: www.lanlangcorp.com
Physical Forms Of Resins
Ion exchange resins come in different physical forms. These forms affect how resins work in water treatment and other processes. The shape and structure impact the resin’s performance and application. Understanding these forms helps choose the right resin for your needs.
Bead Type Resins
Bead type resins are small, round particles. They look like tiny spheres. These beads are uniform in size and shape. This uniformity allows water or other liquids to pass evenly. Bead resins are easy to handle and pack in columns. They offer a large surface area for ion exchange. This type is very common in water softening and purification.
Gel And Macroporous Types
Gel resins have a dense, smooth structure. They swell when in contact with water. This swelling helps ions move inside the beads. Gel resins are good for softening and deionization. Macroporous resins have larger pores inside. These pores allow better flow of water and ions. Macroporous types are more durable in harsh conditions. They are useful for complex water treatment tasks.
Specialty Ion Exchange Resins
Specialty ion exchange resins serve unique purposes beyond basic water softening or deionization. These resins target specific ions or chemicals in water and industrial processes. Their design allows them to handle complex tasks in metal recovery, purification, and chemical separation. Specialty resins offer tailored solutions where conventional resins might fall short.
Chelating Resins
Chelating resins capture metal ions through strong chemical bonds. They contain functional groups that selectively bind metals like copper, iron, and lead. These resins work well in wastewater treatment and metal recovery systems. Their ability to remove trace metals helps protect the environment and improves product quality.
Chelating resins are widely used in mining and plating industries. They efficiently extract valuable metals from complex mixtures. Their selectivity makes them ideal for applications requiring precise metal separation.
Mixed Bed Resins
Mixed bed resins combine cation and anion exchange resins in one unit. This mix provides high purity water by removing both positive and negative ions simultaneously. They are common in laboratories and power plants where ultra-pure water is critical.
Mixed bed resins regenerate fully to restore their ion exchange capacity. Their compact design saves space and simplifies system maintenance. These resins deliver consistent water quality in demanding applications.
Credit: www.netsolwater.com
Choosing The Right Resin
Choosing the right ion exchange resin is key for effective water treatment or chemical processing. The right resin improves efficiency and lowers costs. It depends on many factors, including the type of ions to remove and the water’s condition. Understanding these factors helps select the best resin for your needs.
Factors Affecting Selection
Resin selection depends on the type of ions in the solution. Cation resins remove positive ions like calcium and magnesium. Anion resins target negative ions such as chloride and sulfate. The water’s pH level also affects resin choice. High acidity or alkalinity can damage some resins. Temperature matters because some resins lose strength in hot water. Resin capacity is important for handling the load without frequent replacement. Consider regeneration type—some resins regenerate faster and use less chemical.
Performance Considerations
Check resin efficiency for ion removal. Higher efficiency means better water quality. Look at resin durability to reduce replacement costs. The resin should resist fouling by organics or iron. Fast kinetics improve treatment speed and save time. Resin particle size influences flow rate and pressure drop. Smaller beads give better exchange but can clog faster. Consider compatibility with the system setup to avoid damage. Proper resin choice ensures reliable, long-lasting performance.
Maintenance And Regeneration
Maintenance and regeneration keep ion exchange resins working well. Over time, resins lose their ability to exchange ions. Dirt, minerals, and chemicals build up and block the resin’s surface. Regular care helps restore their performance and extends their life. This section explains common regeneration methods and tips to keep resins effective longer.
Common Regeneration Methods
Regeneration restores the resin’s ion exchange capacity. Different resins need specific chemicals for regeneration. For cation resins, strong acids like hydrochloric acid are common. Anion resins often use strong bases such as sodium hydroxide. The chemicals flush out trapped ions and replace them with fresh ions.
Salt solutions, especially sodium chloride, regenerate softening resins. The process removes hardness ions like calcium and magnesium. After regeneration, resins return to their original state and work properly again. Regular regeneration cycles prevent resin from becoming exhausted or clogged.
Extending Resin Lifespan
Proper care extends resin lifespan. Avoid harsh chemicals that damage resin beads. Monitor water quality to reduce debris and contaminants. Use pre-filters to protect the resin from dirt and particles. Follow recommended regeneration schedules to keep resins active.
Rinse resins thoroughly after regeneration to remove leftover chemicals. Store resins in moist conditions to prevent drying and cracking. Regular inspection helps detect early signs of damage. These steps maintain resin efficiency and reduce replacement costs.
Credit: www.slideserve.com
Frequently Asked Questions
What Are The Main Types Of Ion Exchange Resins?
The main types of ion exchange resins are cation exchange resins and anion exchange resins. Cation resins exchange positive ions, while anion resins exchange negative ions. Both types are widely used in water purification and chemical processing.
How Do Cation And Anion Resins Differ?
Cation resins exchange positively charged ions like calcium and sodium. Anion resins exchange negatively charged ions like chloride and sulfate. Their functional groups and applications vary accordingly, making them suitable for different ion removal processes.
What Are Strong Acid And Weak Acid Cation Resins?
Strong acid cation resins have sulfonic acid groups and work well in all pH ranges. Weak acid cation resins contain carboxylic acid groups and are effective in removing heavy metals in specific pH ranges.
When Should You Use Strong Base Versus Weak Base Anion Resins?
Strong base anion resins remove both strong and weak acids and function in a wide pH range. Weak base anion resins mainly remove weak acids and are better for organic acid removal and regeneration with mild chemicals.
Conclusion
Ion exchange resins come in different types for various uses. Each type works by swapping specific ions to clean or soften water. Choosing the right resin depends on what you need to treat. Understanding these types helps in picking the best option.
This knowledge ensures better results in water treatment or other processes. Simple and clear use of ion exchange resins makes tasks easier and more effective. Keep these basics in mind when working with ion exchange resins.

Hasan Al Sarker is a Reverse Osmosis Specialist. He has worked for many years to ensure safe drinking water for all. His research paper has been published in several journals, including Issue, Medium, and Slideshare. He is recognized as a water doctor among specialists though he did not attend medical college.
Besides working as a researcher of reverse osmosis technology, he is also very fancy with the kitchen and cooking. His guides are reading thousands of people every day. As a head of content, he is responsible for all the published articles at RO System Reviews.
