Have you ever wondered what happens when ion exchange resins lose their power to clean water? These tiny beads work hard to remove unwanted minerals and impurities, but over time, they get exhausted and need a fresh start.
Understanding how exhausted ion exchange resins are regenerated can save you money, improve water quality, and extend the life of your system. You’ll discover simple steps and insider tips to bring your resins back to life efficiently. Keep reading to learn how to make your water treatment process work smarter, not harder.
Credit: dardel.info
Ion Exchange Resin Basics
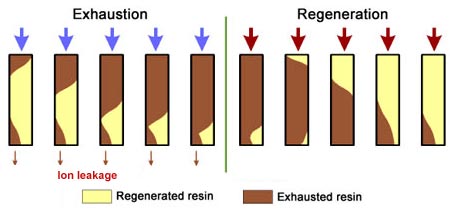
Ion exchange resins are small beads made from organic polymers. They have special sites that swap ions in water. This process helps clean and soften water. Understanding these resins is key to learning how they are regenerated after use.
Resins work by exchanging unwanted ions in water with more desirable ones. Over time, they get full or “exhausted.” At this point, regeneration is needed to restore their function.
Types Of Ion Exchange Resins
There are two main types of ion exchange resins. Cation resins exchange positive ions like calcium and magnesium. Anion resins swap negative ions like chloride and sulfate. Each type targets different impurities in water.
Some resins are strong acid or strong base types. These vary in their ability to handle different water conditions. Choosing the right resin depends on the water treatment goal.
Role Of Resins In Water Treatment
Ion exchange resins remove hardness, salts, and other contaminants. They improve water quality for drinking, industrial use, and more. Softening water protects pipes and appliances from damage.
Resins also help in demineralization and purification processes. They make water safe and clean by removing harmful ions. This role is essential in many water treatment systems.
Signs Of Resin Exhaustion
Exhausted ion exchange resins lose their ability to clean water effectively. Identifying signs of resin exhaustion helps maintain water quality. Early detection avoids costly repairs and downtime. Watch for changes in water taste, smell, or clarity. These signs show the resin needs regeneration or replacement.
Indicators Of Reduced Efficiency
Water flow may slow down noticeably. The resin cannot remove ions well anymore. Hardness or iron levels in water can rise. The treated water may taste salty or metallic. Frequent system backwashing might occur without improvement. These are clear signs the resin is exhausted.
Common Causes Of Resin Exhaustion
High usage without timely regeneration wears out the resin. Exposure to chlorine or strong chemicals damages resin beads. Hard water with high mineral content shortens resin life. Poor maintenance leads to resin clogging and fouling. Understanding these causes helps prevent premature exhaustion.
Regeneration Principles
Exhausted ion exchange resins lose their ability to soften or purify water. Regeneration restores their function. This process removes unwanted ions and replaces them with active ions. It brings the resin back to life for reuse. The principles behind regeneration are both chemical and mechanical. Understanding these principles helps maintain resin efficiency and extends its lifespan.
Chemical Reactions Involved
Regeneration uses specific chemicals to reverse ion exchange. For cation resins, a strong acid like hydrochloric acid or sulfuric acid is common. This acid replaces hardness ions like calcium and magnesium with hydrogen ions.
Anion resins use a strong base, usually sodium hydroxide. The base swaps out unwanted ions such as chloride or sulfate. It restores the resin’s active sites with hydroxide ions.
The chemical reactions depend on the resin type and the ions removed. These reactions clean the resin and prepare it for another cycle of ion exchange.
Regeneration Cycles
The regeneration process follows defined cycles. First, the resin bed is rinsed to remove suspended solids. Then, the regenerant chemical flows through the resin slowly. This flow ensures a complete exchange of ions.
After chemical treatment, the resin is rinsed again to remove excess chemicals. Proper rinsing prevents contamination of treated water. The cycle ends with testing to confirm resin readiness.
Each cycle is controlled by time, flow rate, and chemical concentration. This control ensures the resin regains maximum capacity and performance.
Regeneration Methods
Exhausted ion exchange resins need regeneration to restore their capacity. The process removes the captured ions and prepares the resin for reuse. Several methods exist, each suited to specific resin types and applications. Understanding these methods helps maintain efficiency and extend resin life.
Brine Regeneration
Brine regeneration is common for water softening resins. It uses a concentrated salt solution, usually sodium chloride. The salt solution displaces calcium and magnesium ions from the resin. This process restores the resin’s ability to soften water. Brine regeneration is cost-effective and simple to perform.
Acid And Alkali Regeneration
Strong acid and alkali solutions clean resins used for ion removal. Acid removes cations like calcium and iron. Alkali removes anions such as sulfate and nitrate. This method is common in mixed bed or specialty resins. It effectively restores ion exchange capacity for various industrial uses.
Thermal Regeneration
Thermal regeneration uses heat to restore resin function. It is applied mainly to certain types of resin in large systems. Heat breaks down organic fouling and regenerates the resin structure. This method is less common but useful for specific resin problems. It requires special equipment and careful temperature control.
Step-by-step Regeneration Process
Regenerating exhausted ion exchange resins restores their ability to remove impurities. This process allows resins to be reused multiple times, saving costs and resources. The regeneration involves several key steps that clean and recharge the resin beads. Each step plays a vital role in ensuring the resin works effectively after regeneration.
Preparation And Backwashing
First, prepare the resin bed by stopping the normal flow. Backwashing cleans the resin by flushing out trapped particles. This step removes debris and reclassifies the resin beads. It also helps to prevent channeling during the next steps. Proper backwashing ensures the resin is ready for chemical treatment.
Chemical Application
Next, apply the regenerant chemical solution to the resin. The type of chemical depends on the resin type and contaminants. For example, salt brine is common for softening resins. The chemical replaces the impurities trapped on the resin beads. Allow enough contact time for the reaction to complete fully.
Rinsing And Testing
After chemical treatment, rinse the resin thoroughly with clean water. Rinsing removes excess chemicals and displaced impurities. Check the resin’s performance by testing water quality after regeneration. Repeat rinsing if necessary to meet quality standards. Proper rinsing ensures the resin is safe for use again.
Factors Affecting Regeneration Efficiency
Regenerating exhausted ion exchange resins is crucial for maintaining their performance. Several factors impact how well the regeneration process works. Understanding these factors helps ensure resins regain their full capacity quickly and efficiently.
Each factor influences the chemical reactions and physical changes during regeneration. Small changes can lead to big differences in resin performance. Let’s explore the main factors that affect regeneration efficiency.
Chemical Concentration
The strength of the regenerating chemical matters a lot. Higher concentration can remove more contaminants from the resin. Too weak a solution may leave impurities behind. Too strong can damage the resin beads. Finding the right balance keeps the resin clean and healthy.
Contact Time
The amount of time the resin stays in contact with the regenerating solution is key. Short contact time may not fully restore the resin. Too long can waste chemicals and energy. Proper timing allows the chemicals to work fully without harming the resin.
Temperature And Flow Rate
Temperature affects how fast the regeneration happens. Warmer temperatures usually speed up reactions. Flow rate controls how quickly the regenerant passes through the resin. Too fast means less contact and poor cleaning. Too slow wastes time and resources. Both must be controlled for best results.
Environmental And Safety Considerations
Regenerating exhausted ion exchange resins involves chemicals and processes that affect the environment and safety. Proper care reduces risks to workers and nature. Understanding key safety and environmental steps helps maintain a safer workspace and protects ecosystems.
Handling Regenerant Chemicals
Regenerant chemicals like acids and bases can harm skin and eyes. Workers must wear gloves, goggles, and protective clothing. Store chemicals in labeled containers away from heat and sunlight. Use proper ventilation to avoid inhaling fumes. Follow safety data sheets for safe handling and emergency actions.
Waste Disposal Practices
Waste from resin regeneration contains harmful substances. Dispose of this waste according to local environmental laws. Use licensed hazardous waste facilities for disposal. Avoid pouring waste into drains or soil. Proper disposal prevents pollution and protects water sources and wildlife.

Credit: www.sciencedirect.com
Advancements In Regeneration Techniques
Exhausted ion exchange resins need regeneration to restore their effectiveness. Advances in regeneration techniques help save time, reduce costs, and lower environmental impact. These new methods improve the efficiency and sustainability of resin regeneration. They also make the process easier to control and monitor.
Automated Regeneration Systems
Automated systems control the regeneration process with precision. They monitor flow rates, chemical dosage, and timing automatically. This reduces human error and improves consistency. Operators can track performance remotely using smart devices. Automation also cuts down on chemical waste by using the exact amounts needed. This saves money and protects the environment.
Eco-friendly Alternatives
New eco-friendly methods use less harmful chemicals for regeneration. Some techniques replace traditional acids and bases with biodegradable substances. Others recycle the regenerant chemicals to reduce waste. These methods lower pollution and reduce water usage. They also help companies meet stricter environmental regulations. Eco-friendly regeneration supports long-term sustainability in water treatment.

Credit: www.youtube.com
Frequently Asked Questions
What Is Ion Exchange Resin Regeneration?
Ion exchange resin regeneration restores resin capacity by removing accumulated ions. It uses specific chemical solutions like acids or bases to replace captured ions, enabling the resin to function effectively again.
Why Are Exhausted Ion Exchange Resins Regenerated?
Exhausted resins lose efficiency after ion saturation. Regeneration renews their ion exchange ability, reduces costs, and minimizes environmental waste by extending resin lifespan.
How Is The Regeneration Process Performed?
Regeneration involves flushing resins with a regenerant solution. This solution displaces the captured ions, restoring the resin’s active sites for further use in water treatment.
What Chemicals Are Used In Resin Regeneration?
Common regenerants include sodium chloride for cation resins and sodium hydroxide or acids for anion resins. The choice depends on resin type and specific application needs.
Conclusion
Exhausted ion exchange resins regain their power through careful cleaning. The process removes trapped ions, making resins ready to use again. Proper regeneration saves money and reduces waste. It helps maintain water quality in many industries. Knowing how resins regenerate supports better system care.
Regular checks ensure the resins work efficiently. Clear steps and simple chemicals restore resin function. This keeps equipment running smoothly and water pure. Understanding this process makes maintenance easier for everyone.

Hasan Al Sarker is a Reverse Osmosis Specialist. He has worked for many years to ensure safe drinking water for all. His research paper has been published in several journals, including Issue, Medium, and Slideshare. He is recognized as a water doctor among specialists though he did not attend medical college.
Besides working as a researcher of reverse osmosis technology, he is also very fancy with the kitchen and cooking. His guides are reading thousands of people every day. As a head of content, he is responsible for all the published articles at RO System Reviews.

