Have you ever wondered what makes ion exchange resin such a powerful tool in water purification and other important processes? Understanding what ion exchange resin is made of can give you a clearer picture of how it works and why it’s so effective.
Whether you’re curious about improving your water quality or just want to know more about this fascinating material, this article will break it down for you in simple terms. Keep reading to discover the secrets behind ion exchange resin and how it can benefit you.
Credit: puretecwater.com
Basic Composition
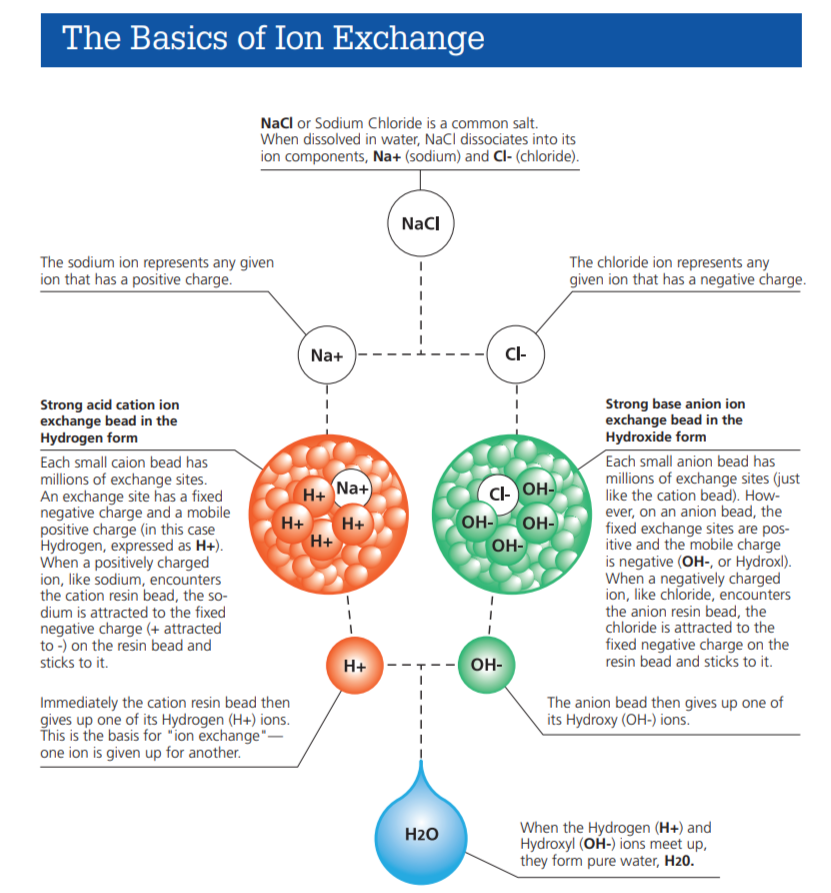
Ion exchange resins are tiny beads made for cleaning water and other liquids. They work by swapping unwanted ions with useful ones. To understand how they work, it helps to know what they are made of. The basic composition includes a strong base and special active parts. These parts work together to hold and exchange ions effectively.
Polymer Matrix
The polymer matrix forms the resin’s solid structure. It is made from long chains of molecules called polymers. These chains create a stable, porous network inside each bead. This network allows liquids to flow through easily. The matrix is tough and keeps the resin shape during use. Common polymers include polystyrene and divinylbenzene. These materials resist chemicals and temperature changes well.
Functional Groups
Functional groups are the active parts of the resin. They attach to the polymer matrix and do the ion exchange work. These groups have charged sites that attract ions from liquids. There are two main types: cation and anion exchange resins. Cation resins hold positive ions like calcium or magnesium. Anion resins catch negative ions like chloride or sulfate. The type of functional group controls which ions the resin can swap.

Credit: samcotech.com
Types Of Functional Groups
Ion exchange resins work by swapping ions with the solution around them. The key to this process lies in their functional groups. These groups attach to the resin’s base and determine which ions the resin can exchange. There are two main types of functional groups in ion exchange resins. Each type plays a different role in purification and water treatment.
Cation Exchange Groups
Cation exchange groups attract and hold positively charged ions. These ions include calcium, magnesium, and sodium. The most common cation exchange group is the sulfonic acid group (-SO3H). It is strong and works well in many water softening applications. Another type is the carboxylic acid group (-COOH). It is weaker but useful in specific cases, such as removing heavy metals. Cation exchange resins release hydrogen ions (H+) or sodium ions (Na+) during the exchange process.
Anion Exchange Groups
Anion exchange groups capture negatively charged ions. These ions include chloride, nitrate, and sulfate. The most typical anion exchange groups are amine groups (-NH2) and quaternary ammonium groups (-NR4+). Quaternary ammonium groups are strong and effective in removing a wide range of anions. Amine groups are weaker and used for selective ion removal. Anion exchange resins release hydroxide ions (OH-) or chloride ions (Cl-) in the exchange process.
Resin Structure
The structure of ion exchange resin plays a crucial role in its function. It consists of a solid matrix that holds ion exchange sites. This matrix is typically made from synthetic polymers. The design allows ions to move in and out efficiently. Understanding the resin structure helps explain its durability and performance.
Crosslinking Agents
Crosslinking agents connect polymer chains inside the resin. These agents create a network that holds the resin’s shape. They control the resin’s strength and swelling. Too little crosslinking makes the resin weak and soft. Too much limits ion movement and reduces efficiency. The right balance helps the resin work well in water treatment and other uses.
Bead Formation
Ion exchange resins come as small beads. These beads provide a large surface area for ion exchange. Bead size affects the flow of water and ions. Uniform beads allow smooth water flow and better ion contact. Beads are made by polymerizing liquid mixtures into solid spheres. This process ensures consistent shape and size for better performance.
Credit: www.netsolwater.com
Material Variations
Ion exchange resins come in different materials, each suited for specific uses. Understanding these material variations helps choose the right resin for water treatment or other processes. The main types differ in origin and chemical strength. These differences affect how well they perform in removing unwanted ions from liquids.
Natural Vs Synthetic Resins
Natural resins come from plant-based sources. They are biodegradable and eco-friendly but less durable. Synthetic resins are made from polymers like polystyrene. They offer higher strength and longer life. Synthetic types dominate industrial uses due to their stability and efficiency. Natural resins suit smaller or less demanding applications.
Strong Vs Weak Ion Exchangers
Strong ion exchangers work in a wide pH range. They can remove a broad spectrum of ions. Weak ion exchangers have a limited pH operating range. They target specific ions, usually with less harsh chemicals. Strong exchangers are common in water softening and purification. Weak exchangers fit well in delicate or specialized treatments.
Manufacturing Process
The manufacturing process of ion exchange resin is a careful blend of science and precision. It starts with creating the base material, which forms the backbone of the resin. This base is then chemically modified to allow the resin to exchange ions effectively. The process involves two main steps: polymerization and functionalization. Each step plays a critical role in determining the resin’s strength and performance.
Polymerization Methods
Polymerization is the first step in making ion exchange resin. It involves linking small molecules called monomers to form a large, solid polymer. This polymer creates tiny beads that serve as the resin’s structure. There are two common methods: suspension and emulsion polymerization. Suspension polymerization produces uniform beads, ideal for ion exchange. The process uses water to suspend the monomers while they react and form beads. These beads are durable and have a high surface area, necessary for effective ion exchange.
Functionalization Techniques
Functionalization adds special groups to the polymer beads. These groups give the resin its ion exchange ability. The process involves chemical reactions that attach acidic or basic groups to the beads. Common functional groups include sulfonic acid, carboxylic acid, and amine groups. The choice depends on the type of ions the resin will target. This step is crucial because it controls how well the resin attracts and holds ions from solutions.
Applications Based On Composition
Ion exchange resins have different uses depending on their makeup. Their composition affects how well they work in various fields. These resins can be made to attract specific ions. This makes them useful for many industrial and medical tasks.
Water Treatment
Ion exchange resins remove unwanted minerals from water. They soften hard water by swapping calcium and magnesium ions. Some resins target heavy metals like lead and mercury. This makes water safer to drink and use in homes.
In large systems, resins clean water for factories and power plants. They help prevent scale buildup and corrosion in pipes. This keeps equipment running longer and saves money.
Pharmaceuticals
In medicine, ion exchange resins help purify drugs. They remove impurities during the manufacturing process. Some resins control the release of medicines in the body. This improves how drugs work and their safety.
Resins are also used in dialysis machines. They filter waste from the blood when kidneys fail. Their precise ion exchange ensures proper treatment for patients.
Food Industry
Ion exchange resins play a key role in food processing. They remove bitter tastes from sugar and juices. This improves flavor and quality of the final product.
Resins also help separate proteins and vitamins. This aids in making supplements and fortified foods. Their use ensures food meets health and safety standards.
Frequently Asked Questions
What Materials Make Up Ion Exchange Resin?
Ion exchange resin is primarily made of synthetic polymers, usually polystyrene or polyacrylic beads. These beads are functionalized with ion-exchange groups like sulfonic acid or quaternary ammonium. This structure allows the resin to exchange ions effectively in water treatment or chemical processes.
How Does The Resin’s Chemical Structure Affect Performance?
The resin’s chemical structure determines its ion selectivity and capacity. Functional groups attached to the polymer backbone enable specific ion exchange. This affects how well the resin removes unwanted ions or adds beneficial ones in applications such as water softening or purification.
Are There Different Types Of Ion Exchange Resins?
Yes, there are two main types: cation and anion exchange resins. Cation resins exchange positively charged ions, while anion resins exchange negatively charged ions. The choice depends on the specific ions targeted for removal or replacement in a process.
What Role Do Cross-linking Agents Play In Resin?
Cross-linking agents provide structural stability to ion exchange resins. They link polymer chains, enhancing mechanical strength and chemical resistance. This ensures the resin maintains its shape and functionality during repeated ion exchange cycles.
Conclusion
Ion exchange resin consists mainly of a polymer base and charged groups. These parts work together to swap ions in liquids. This process helps clean water and treat chemicals. Understanding what resins are made of shows why they are useful.
They come in different types for various tasks. Knowing their materials aids in choosing the right resin. This makes ion exchange a practical solution in many fields. Simple yet effective.

Hasan Al Sarker is a Reverse Osmosis Specialist. He has worked for many years to ensure safe drinking water for all. His research paper has been published in several journals, including Issue, Medium, and Slideshare. He is recognized as a water doctor among specialists though he did not attend medical college.
Besides working as a researcher of reverse osmosis technology, he is also very fancy with the kitchen and cooking. His guides are reading thousands of people every day. As a head of content, he is responsible for all the published articles at RO System Reviews.

